Petroleum coke, abbreviated coke or petcoke, is a final carbon-rich solid material derived from oil refining and is one type within the group of fuels known as cokes. Petcoke specifically comes from a final cracking process—a thermo-based chemical engineering process that breaks long-chain hydrocarbons in petroleum into shorter chains—conducted in units known as coker units. (Other types of coke are derived from coal.)
In brief, coke is the carbonization product of high-boiling hydrocarbon fractions obtained from processing heavy petroleum residues. Petcoke is also produced during the generation of synthetic crude oil (syncrude) from bitumen extracted from Canada’s oil sands and Venezuela’s Orinoco oil sands.
In petroleum coker units, residual oils from other refining distillation processes are treated under high temperature and pressure. This drives off gases and volatiles and separates the remaining light and heavy oils, leaving behind the petcoke. These are known as coking processes, most commonly utilizing delayed coking operations in chemical engineering plant systems.
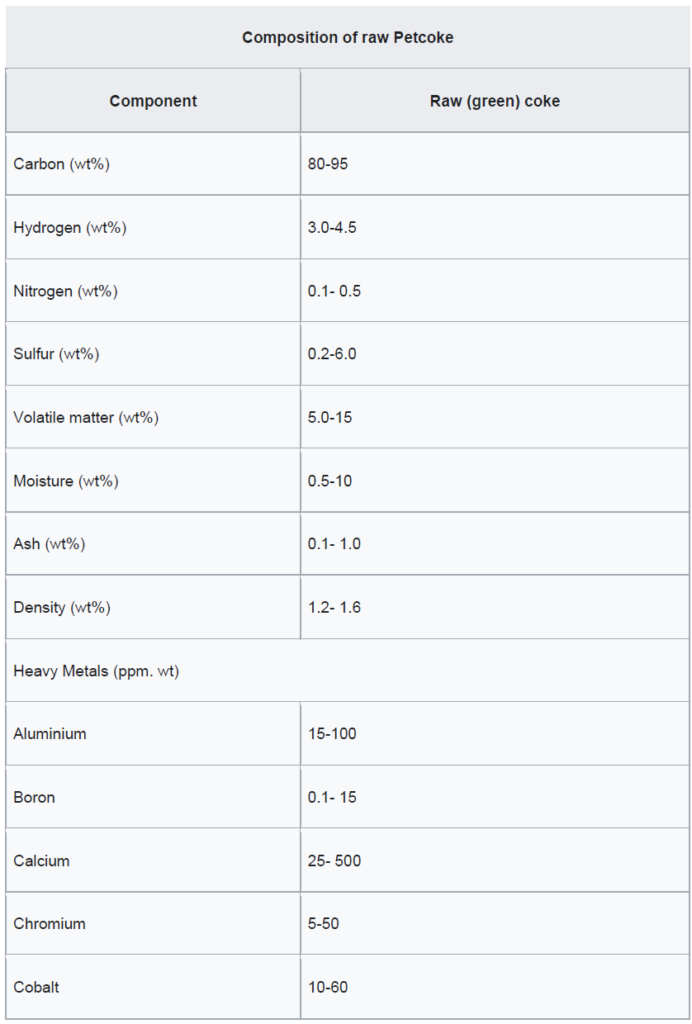
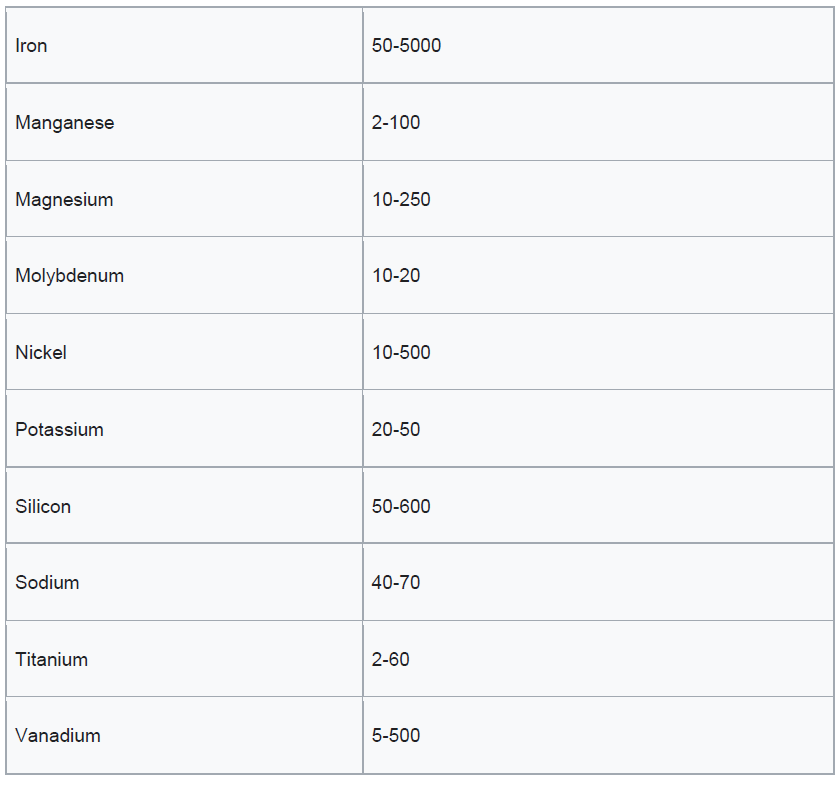
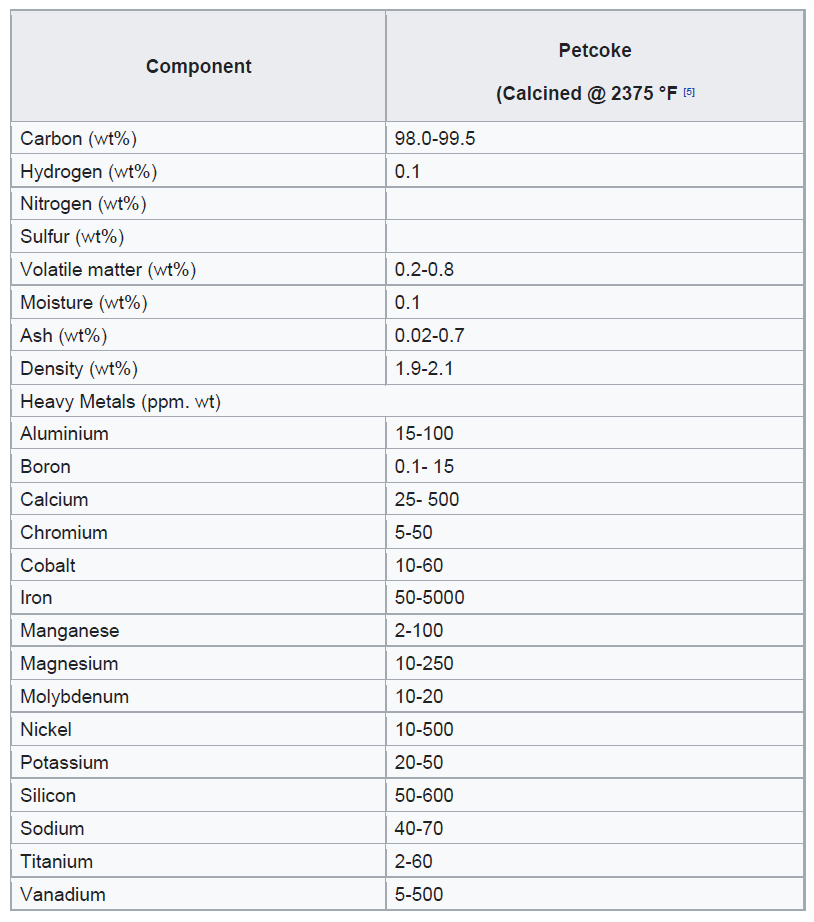
This coke can be categorized as either fuel grade (high in sulfur and metals) or anode grade (low in sulfur and metals). The raw coke taken directly from the coker is referred to as green coke. In this context, “green” means unprocessed.
Further processing of green coke through calcining in a rotary kiln removes residual volatile hydrocarbons. The resulting calcined petroleum coke can then be processed in an anode baking oven to produce anode coke with specific shape and physical properties. These anodes are primarily used in the aluminium and steel industries.
Petcoke consists of over 80% carbon and emits 5% to 10% more carbon dioxide (CO₂) than coal on a per-unit-of-energy basis when burned. Due to its higher energy content, petcoke emits 30% to 80% more CO₂ than coal on a per-unit-of-weight basis.
The difference in CO₂ production per unit of energy between coal and coke depends on:
The amount of volatile hydrocarbons in both coal and coke, which tends to decrease CO₂ emissions per unit of energy.
The moisture content in coal, which increases CO₂ emissions per unit of energy (heat of combustion).